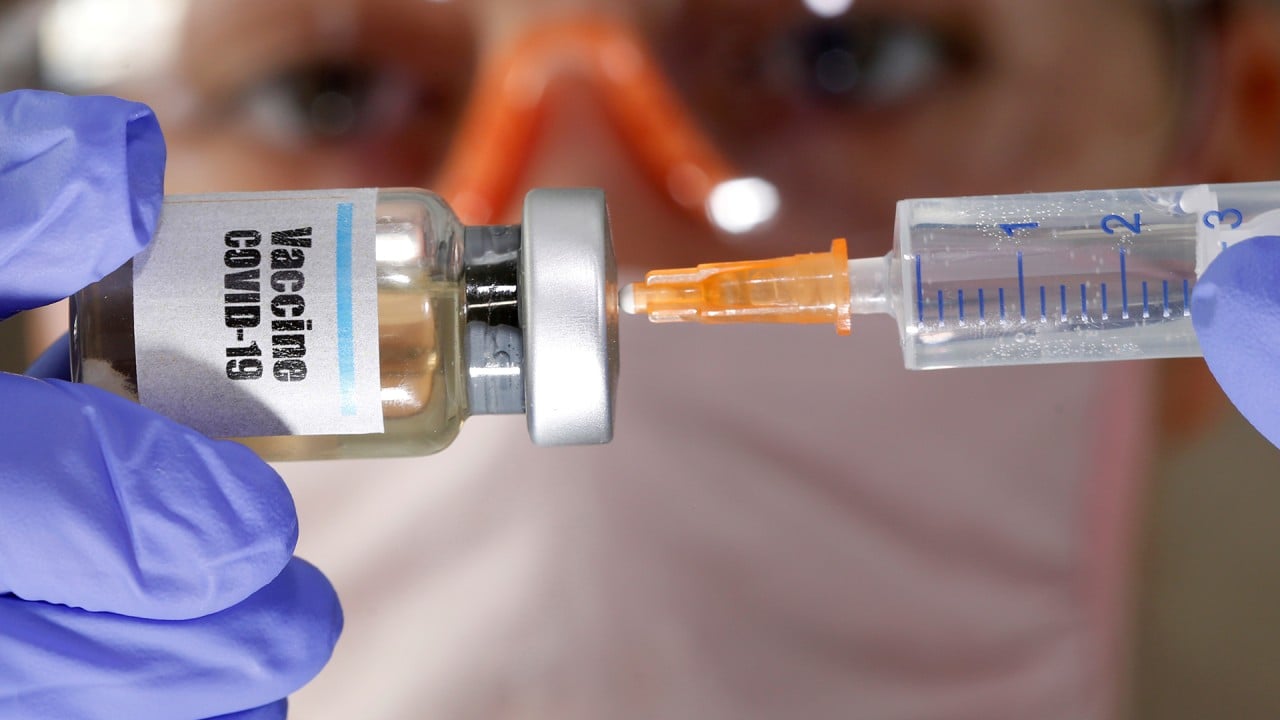
Coronavirus: Fosun Pharma, BioNTech’s China vaccine trial is an important step before securing marketing approval, companies say
- 144 participants in mainland China will receive two shots of the BNT162b1 coronavirus vaccine candidate spaced by 21 days
- BioNTech among the first international biopharmaceutical companies to start clinical trial of a Covid-19 vaccine candidate in China

The first 72 of 144 participants in mainland China received a dose of BNT162b1, according to a statement from the companies.
“We are proud to be among the first international biopharmaceutical companies to initiate a clinical trial of a Covid-19 vaccine candidate in China as part of our effort to make our vaccine available globally, if approved,” said Ugur Sahin, chief executive officer and co-founder of BioNTech.
The beginning of the clinical trial is an “important step” for the companies to secure authorisation to market the vaccine, he added.
06:17
‘Robust immune responses’ found in Covid-19 vaccine clinical trials point to 2021 release
The trial will enrol 144 healthy individuals to evaluate the safety and effectiveness of the vaccine and to confirm dose selection, the companies said.
The subjects will be divided into two groups, one comprising of healthy adults aged 18 to 55, and the other, comprising those over 55 years old. Both groups will receive two injections of varying doses at 21 days apart. The participants will receive their shots in Taizhou, Jiangsu province.
